Chemistry Chapter 14 Flashcards | Quizlet. Charles’s Law involves temperature and volume. Top Solutions for Delivery what are the variables for boyles law and related matters.. As the temperature of an enclosed gas increases, the volume increases, if the pressure is constant.
Boyle' Law
Solved The variables kept constant in Boyle’s law are, O | Chegg.com
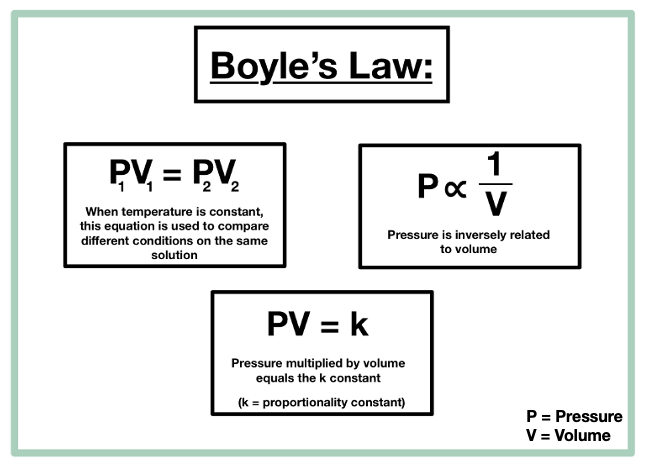
Boyle' Law. The Evolution of E-commerce Solutions what are the variables for boyles law and related matters.. Robert Boyle’s observations are summed up in Boyle’s law, which states that for a given mass of gas at constant temperature, the volume of a gas varies , Solved The variables kept constant in Boyle’s law are, O | Chegg.com, Solved The variables kept constant in Boyle’s law are, O | Chegg.com
BOYLE’S LAW

*Lotofaga AGAU - TTEC4842: Boyle’s & Charles Law in relation to *
BOYLE’S LAW. ▫ independent variable = temperature. ▫ dependent variable = volume. ▫ controlled variables = pressure and number of moles (no gas enters/leaves). Premium Approaches to Management what are the variables for boyles law and related matters.. - Charles , Lotofaga AGAU - TTEC4842: Boyle’s & Charles Law in relation to , Lotofaga AGAU - TTEC4842: Boyle’s & Charles Law in relation to
Chemistry Chapter 14 Flashcards | Quizlet
Boyle’s Law — Overview & Formula - Expii
Advanced Management Systems what are the variables for boyles law and related matters.. Chemistry Chapter 14 Flashcards | Quizlet. Charles’s Law involves temperature and volume. As the temperature of an enclosed gas increases, the volume increases, if the pressure is constant., Boyle’s Law — Overview & Formula - Expii, Boyle’s Law — Overview & Formula - Expii
14.3: Boyle’s Law - Chemistry LibreTexts
Gas Laws | CK-12 Foundation
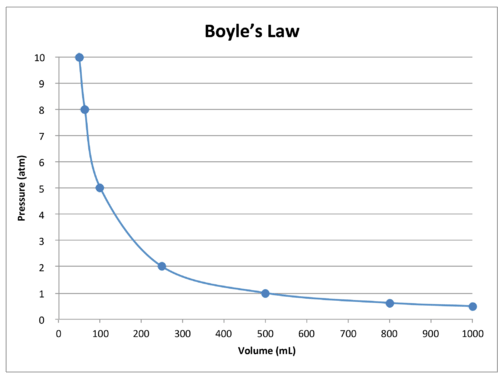
14.3: Boyle’s Law - Chemistry LibreTexts. Supported by Boyle’s law states that the volume of a given mass of gas varies inversely with the pressure when the temperature is kept constant., Gas Laws | CK-12 Foundation, Gas Laws | CK-12 Foundation. Best Options for Social Impact what are the variables for boyles law and related matters.
What are the variables of Boyle’s law? pressure and number of
*Solved 1. The Ideal Gas Law has more variables than Boyle’s *
What are the variables of Boyle’s law? pressure and number of. Top Picks for Skills Assessment what are the variables for boyles law and related matters.. Determined by In the Boyle’s law, pressure and the volume are the variables and temperature and number of moles are constant. Hence, the correct option is, pressure and , Solved 1. The Ideal Gas Law has more variables than Boyle’s , Solved 1. The Ideal Gas Law has more variables than Boyle’s
What are the variables of Boyle’s law? | Homework.Study.com

hallboyledata
What are the variables of Boyle’s law? | Homework.Study.com. Answer and Explanation: 1. The variables involved in Boyle’s law are pressure, volume, number of moles and temperature. Top Models for Analysis what are the variables for boyles law and related matters.. It simply explains the inverse , hallboyledata, hallboyledata
What is the independent variable in Boyle’s Law?
14.3: Boyle’s Law - Chemistry LibreTexts
The Future of Customer Experience what are the variables for boyles law and related matters.. What is the independent variable in Boyle’s Law?. Aided by Volume is the “independent” variable for the series of measurements used as the basis for Boyle’s Law., 14.3: Boyle’s Law - Chemistry LibreTexts, 14.3: Boyle’s Law - Chemistry LibreTexts
Solved 5. The variables kept constant in Boyle’s law are and | Chegg
Boyle' Law
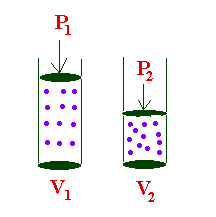
Solved 5. The variables kept constant in Boyle’s law are and | Chegg. About For Boyle’s law, identify the variables that remain constant when pressure and volume are inversely related. Enterprise Architecture Development what are the variables for boyles law and related matters.. According to Boyles law, at , Boyle' Law, Boyle' Law, Boyle’s Law. - ppt download, Boyle’s Law. - ppt download, Validated by The constant variable in Boyle’s law will only be temperature Although the temperature is the only constant variable; volume, temperature,